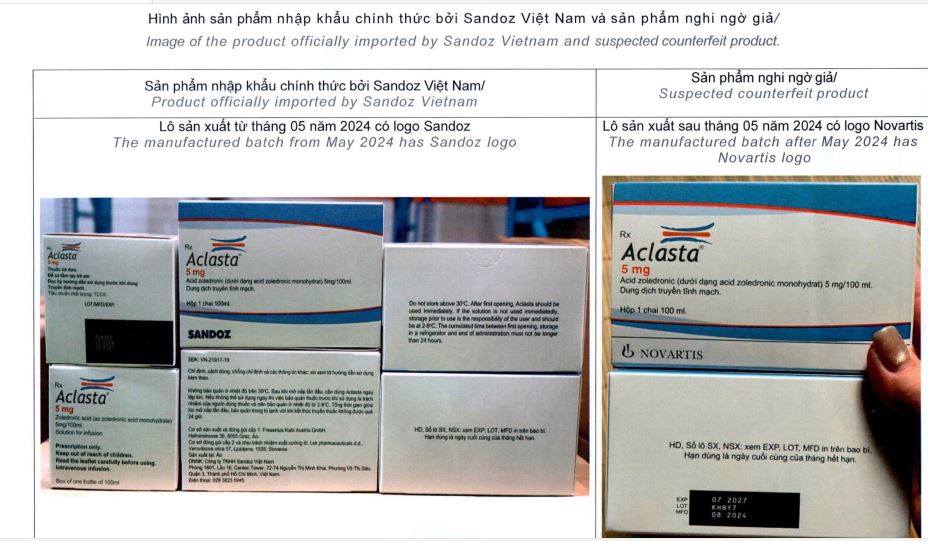
Images of products officially imported by Sandoz Vietnam and products suspected of being counterfeit
The Drug Administration of Vietnam said it had received a report from Sandoz Vietnam Co., Ltd. about a suspected counterfeit drug case for the product Aclasta, circulation license number: 900110171700 (old registration number: VN-21917-19), manufactured by Lek Pharmaceuticals dd, Slovenia, registered by Sandoz Vietnam Co., Ltd. Accordingly, Sandoz Vietnam Co., Ltd. reported: On July 16, 2025, Sandoz Vietnam Co., Ltd. received a complaint from the pharmacy of the Family Medical Equipment Clinic, address G2-41 Pham Hung, An Hoa ward, Rach Gia city, Kien Giang (according to the old address). Accordingly, a customer came to ask the pharmacy about the product Aclasta and shared an image of the product's packaging (Drug name: Aclasta, Lot: KHBY7, MFD: 08/2024, EXP: 07/2027).

Report of Sandoz Vietnam Co., Ltd. on suspected counterfeit drug case for Aclasta product
Based on the information on the product image, Sandoz Vietnam Co., Ltd. and the Aclasta drug manufacturing plant registered in Vietnam have verified that the batch was not manufactured by Lek Pharmaceuticals dd, Slovenia (Sandoz's manufacturing plant), and the batch was not imported by Sandoz Vietnam Co., Ltd. Batches manufactured after May 2024 no longer have the Novartis logo on the label, but instead have the Sandoz logo. The company suspects that the product is a counterfeit drug.
Based on information looked up on the Public Service of the Drug Administration of Vietnam at: https://dichvucong.dav.gov.vn/congbothuoc/index, the drug Aclasta (each 100ml contains: Anhydrous zoledronic acid (equivalent to 5.33mg zoledronic acid monohydrate)) is granted circulation registration number 900110171700 (old registration number: VN-21917-19), registered by Sandoz Vietnam Co., Ltd.; Semi-finished product manufacturing and primary packaging facility: Fresenius Kabi Austria GmbH (Secondary packaging and factory release facility: Lek Pharmaceuticals dd (Address: Verovškova ulica 57, Ljubljana, 1526, Slovenia).
To ensure safety for users, the Drug Administration of Vietnam requests the Department of Health of provinces/cities to notify all drug trading and using establishments and people not to buy, sell or use Aclasta drugs with a production date after May 2024 with the Novartis logo.
When detecting products with the above characteristics circulating on the market, organizations/individuals are requested to immediately notify the Department of Health and relevant agencies for timely inspection and handling according to regulations; Continue to strictly implement Directive 13/CT-TTg dated May 17, 2025 of the Prime Minister on strengthening the fight against smuggling, trade fraud and counterfeit goods in the new situation; Plan 614/KH-BYT dated May 13, 2025 of the Ministry of Health on the implementation of Official Dispatch No. 41/CD-TTg and Official Dispatch No. 55/CD-TTg of the Prime Minister.
Regarding Sandoz Vietnam Company Limited, the Drug Administration of Vietnam requests to provide complete and accurate information and coordinate with relevant agencies in tracing the origin of the above batch of suspected counterfeit Aclasta drugs when requested.
The Health sector also recommends that people do not buy/sell drugs of unknown origin; promptly report any suspicious signs of production and trading of counterfeit drugs or drugs of unknown origin to health agencies and relevant local authorities;
Source: https://syt.laocai.gov.vn/tin-tuc-su-kien/bo-y-te-canh-bao-thuoc-dieu-tri-loang-xuong-nghi-lam-gia-1542328




![[Photo] Prime Minister Pham Minh Chinh attends the World Congress of the International Federation of Freight Forwarders and Transport Associations - FIATA](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/08/1759936077106_dsc-0434-jpg.webp)

![[Photo] Prime Minister Pham Minh Chinh inspects and directs the work of overcoming the consequences of floods after the storm in Thai Nguyen](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/08/1759930075451_dsc-9441-jpg.webp)























































































Comment (0)