On September 16, a representative of the Drug Administration said that Novartis Vietnam had received four complaints from users about suspected counterfeit eye drop products, including Tobrex, Maxitrol, and TobraDex. Through verification, Tobrex 5 ml lot number VEE90A was confirmed to be counterfeit. Three other lots, including Tobrex 5 ml (lot VEE98C), Maxitrol 5 ml (lot VFD09A), and TobraDex 5 ml (lot VHN07A), were also suspected of being counterfeit. These products were all found circulating outside the company's official distribution system. A representative of the Drug Administration said that the above products were widely sold on the market.
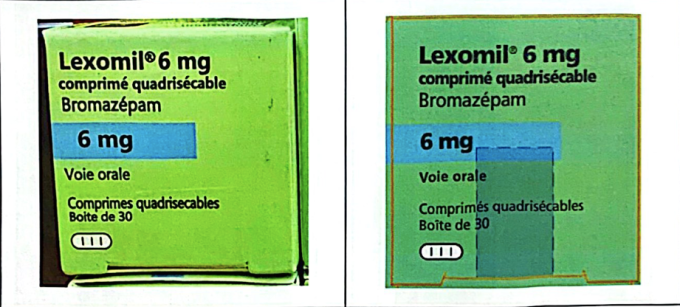
The second case is the high-dose sleeping pill Lexomil 6 mg, owned by Cheplapharm Arzneimottel GmbH. Ho Chi Minh City police seized batch number F3193F01, expiry date December 2027, and confirmed by the manufacturer to be counterfeit. This batch was originally manufactured for circulation in the French market, not imported into Vietnam. Currently, Lexomil 6 mg does not have a domestic circulation registration number, so all trading and use of this product is illegal.
Finally, there is the drug Aclasta (containing zoledronic acid), used in the treatment of osteoporosis. A pharmacy in Kien Giang reported a product with a production date of August 2024 and an expiration date of July 2027. The registered company confirmed that this batch of drugs was not officially imported and the packaging had abnormalities. Specifically, genuine Aclasta drugs manufactured after May 2024 have replaced the Novartis logo with the Sandoz logo. Therefore, products manufactured after this time but still carrying the old logo are suspected to be fake.
In this situation, the Drug Administration requested local health departments to notify hospitals, pharmacies and people not to trade or use the above drugs. Units were assigned to urgently inspect, monitor and trace the origin of violating products.
The management agency recommends that people carefully check the information on the packaging and compare it with the licensed drug data on the Drug Administration's portal. When detecting suspicious signs, people should immediately report to the authorities for timely handling.
PV (synthesis)Source: https://baohaiphong.vn/bo-y-te-canh-bao-khan-3-loai-thuoc-bi-nghi-lam-gia-521002.html




![[Photo] Closing ceremony of the 18th Congress of Hanoi Party Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/17/1760704850107_ndo_br_1-jpg.webp)





























![[Photo] Nhan Dan Newspaper launches “Fatherland in the Heart: The Concert Film”](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/16/1760622132545_thiet-ke-chua-co-ten-36-png.webp)




































































Comment (0)