The Ministry of Health informs about some new points in Decree No. 96 of the Government detailing a number of articles of the Law on Medical Examination and Treatment.
Accordingly, on January 9, 2023, at the 2nd Extraordinary Session of the 15th National Assembly, the amended Law on Medical Examination and Treatment was passed. The National Assembly assigned the Government, the Ministry of Health and relevant Ministries and Branches to develop Decrees, circulars and documents detailing and implementing the Law on Medical Examination and Treatment.
From January 1, 2024, the Law on Medical Examination and Treatment and its guiding documents will take effect, including a number of new contents from the perspective of taking patients as the center, improving the quality of medical examination and treatment.
The Government has issued Decree No. 96 detailing a number of articles of the Law on Medical Examination and Treatment, effective from January 1, 2024. A number of important contents have been detailed, including:
New regulations on granting medical practice licenses and managing medical practice, specifying the contents of practical guidance in medical examination and treatment, conditions, records, procedures for granting new, re-granting, extending, adjusting medical practice licenses and registering to practice in medical examination and treatment, and activities of the National Medical Council in examining and assessing the capacity of practitioners.
Practice in medical examination and treatment has changed, including shortening the practice time for doctors from 18 months to 12 months, and for nurses, midwives, and medical technicians from 9-12 months to 6-9 months, with specific regulations on practice content.
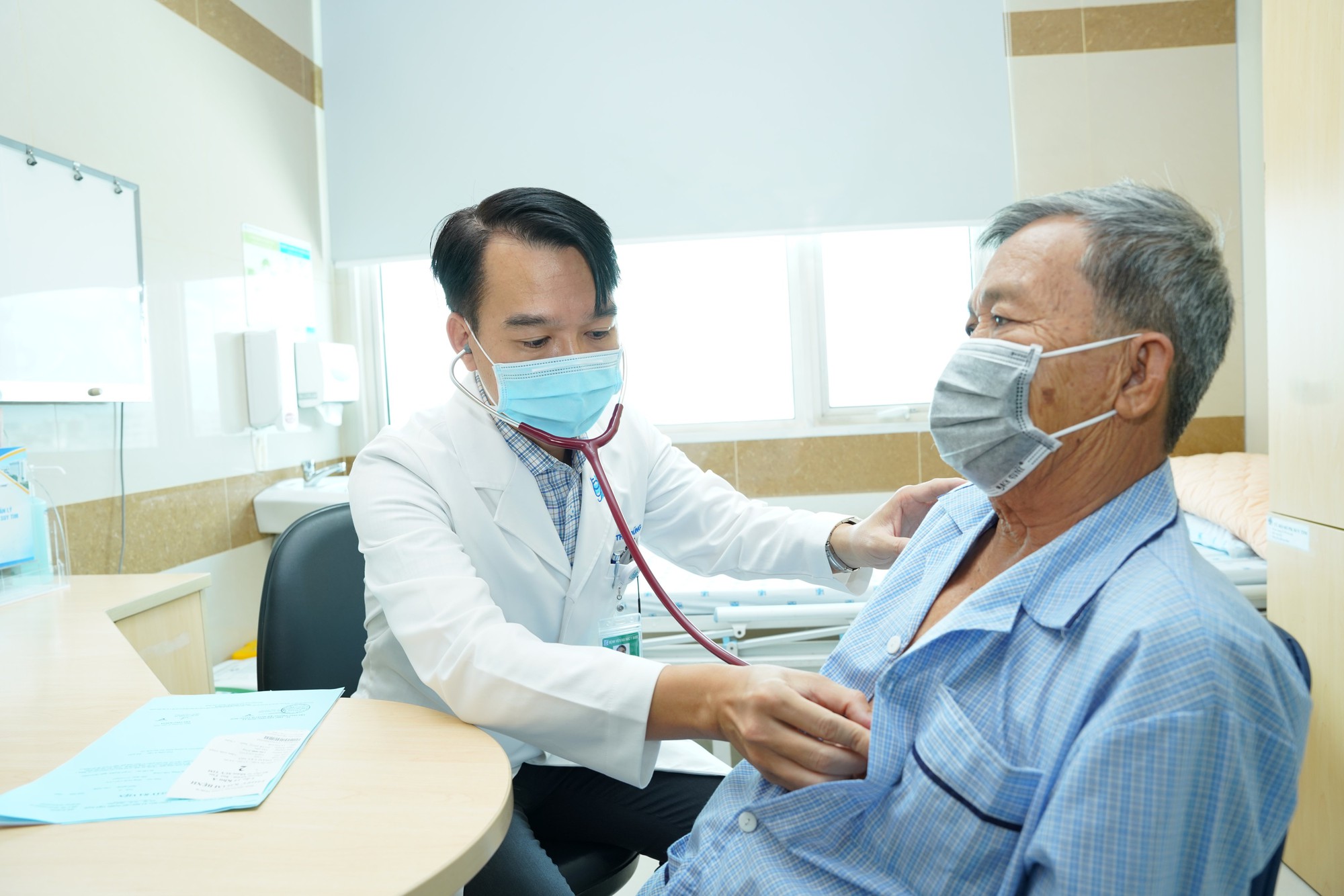
Detailing a number of articles of the Law on Medical Examination and Treatment.
The dossiers for new issuance, re-issuance, extension and adjustment of practice licenses have been reduced and simplified, including the removal of the judicial record in the dossier, the replacement of personal records with autobiographical records, and the mandatory confirmation of the commune-level People's Committee in the records.
Competency assessment tests will be conducted from January 1, 2027 for doctors, and from January 1, 2028 for physicians, nurses, midwives, and medical technicians.
With 3 new professional titles including: clinical nutritionist, outpatient emergency worker, clinical psychologist, the practice, conditions, records, and licensing procedures have been specifically regulated to issue practice licenses from January 1, 2024, and participate in the assessment of professional capacity from January 1, 2029 according to the Law's roadmap.
During the period from the effective date of the Law to the time of the assessment of professional competence, subjects eligible for a practice license will be granted a license without having to undergo a capacity assessment test. Practitioners who have been granted a practice certificate before will not have to undergo a capacity assessment test according to the provisions of the Law.
Regarding the issuance of operating licenses and management of medical examination and treatment facilities, a number of forms of medical examination and treatment facilities have been supplemented and adjusted, including a number of new types of medical examination and treatment facilities such as: medical doctor clinics, physician clinics, interdisciplinary clinics, family medicine medical examination and treatment facilities, optical facilities that perform refractive error testing and measurement, blood filtration facilities, etc. The conditions, documents, and procedures for issuing new, re-issuing, and adjusting operating licenses have been adjusted, removing a number of difficulties and at the same time solving needs and meeting practical requirements, creating conditions for the development of medical examination and treatment facilities, reducing business conditions, and simplifying administrative procedures.
Remote medical examination and treatment, humanitarian medical examination and treatment, and mobile medical examination and treatment have been specifically regulated. In particular, specific regulations on conditions and list of diseases eligible for remote medical examination and treatment are a new point in the Law on Medical Examination and Treatment that has been specified.
The technical expertise classification for medical examination and treatment facilities has been regulated with specific and detailed assessment criteria according to the Law on Medical Examination and Treatment. The highlight of the provisions in the Law on Medical Examination and Treatment and Decree No. 96 is the technical expertise classification of medical examination and treatment facilities based on professional capacity, technical support capacity, practical training capacity and scientific research capacity. The technical expertise classification does not depend on the administrative level but is entirely based on the professional capacity of the medical examination and treatment facility.
Regulations related to the assessment of the quality of medical examination and treatment facilities and the organization certifying the quality of medical examination and treatment facilities are the legal basis for assessing the quality of medical examination and treatment facilities according to basic quality standards, advanced quality standards, quality standards for each specialty or each technical service, creating conditions for the application and recognition of international or foreign quality standards, the establishment of independent quality certification organizations, contributing to promoting the improvement of the quality of medical examination and treatment.
Detailed regulations on processes, records and procedures related to the application of new techniques and new methods: Implementing the Law on Medical Examination and Treatment, the Decree also detailed regulations on processes, records and procedures related to the application of new techniques and new methods in medical examination and treatment, in which, according to the provisions of the Law, there are only 2 types of new techniques and new methods, which are techniques and methods applied for the first time in Vietnam or applied for the first time in the world.
Compared with the provisions of the 2009 Law on Medical Examination and Treatment, the new provisions have limited the scope to only 2 groups of new techniques and new methods compared to the previous 3 groups (including new techniques and new methods for medical examination and treatment facilities).
Thus, according to the provisions of the Law and Decree, medical examination and treatment facilities applying techniques for the first time at that facility, if they do not belong to the group of techniques applied for the first time in the world or in Vietnam, will only apply the procedure of adding the list of techniques or apply the regulations on technology transfer, the procedures have been simplified compared to previous regulations.
Regulations related to clinical trials of new techniques, new methods, and clinical trials of medical equipment have also been specifically regulated, creating a legal corridor for the introduction of new techniques, new methods, and new medical equipment into Vietnam or researched and developed in Vietnam with strict processes, records, and procedures, applied in accordance with international practices.

The Ministry of Health plans to abolish 92 administrative procedures and issue 34 new administrative procedures.
Decree regulating the management of medical equipment at medical examination and treatment facilities, specifically regulating the principles of management and use of medical equipment in medical examination and treatment facilities, requirements for management, use, inspection, maintenance, repair, replacement of materials and components, inspection and calibration of medical equipment.
The Decree also allows for priority processing of some cases of medical equipment circulation registration dossiers as prescribed in Decree No. 98 dated November 8, 2021 of the Government on medical equipment management to help speed up and ensure supply for medical examination and treatment.
One of the contents added to the Law and Decree is the issue of mobilizing and dispatching medical examination and treatment facilities to participate in medical examination and treatment activities in case of natural disasters, catastrophes, group A infectious diseases and emergencies. These are also regulations to remove obstacles and difficulties that have occurred in the practice of fighting the Covid-19 pandemic in recent years, concretizing Resolution No. 30 of the National Assembly and Resolution No. 12 of the National Assembly Standing Committee.
Regarding the conditions for ensuring the operation of medical examination and treatment facilities. Conditions related to liability insurance, financial regulations, support mechanisms for a number of priority subjects, and socialization have been specified in some contents.
Specific regulations on prices for medical examination and treatment services, funding for out-of-hospital emergency operations, some costs in care, medical examination, treatment, and care for patients without relatives, and deceased people with no one to receive them at medical examination and treatment facilities.
In addition to Decree No. 96, which the Ministry of Health advised the Government to issue, the Ministry of Health has also issued circulars specifying a number of articles of the Law on Medical Examination and Treatment (Circulars No. 27, 28, 30, 32, 34) to specify a number of contents assigned by the Law. Regulations related to the scope of practice, continuous updating of medical knowledge, activities of village health workers, village midwives, health workers of units, agencies, organizations, content of recognition of quality standards for medical examination and treatment facilities, organization and operation of professional councils in dispute resolution in medical examination and treatment, regulations on medical record forms.
Law on Medical Examination and Treatment No. 15 and its guiding documents have resolved many difficulties and problems for practitioners, medical examination and treatment facilities, mobilization mechanisms in epidemic prevention, socialization issues, service prices and conditions to ensure the operation of medical examination and treatment facilities.
According to the Ministry of Health, with the patient-centered perspective, the Law and guiding documents have promoted and improved the quality of medical examination and treatment.
The Ministry of Health plans to abolish 92 administrative procedures, issue 34 new administrative procedures, and replace 3 administrative procedures to implement Decree No. 96 .
Source



![[Photo] Keep your warehouse safe in all situations](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/1/3eb4eceafe68497989865e7faa4e4d0e)
![[Photo] Hanoi morning of October 1: Prolonged flooding, people wade to work](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/1/189be28938e3493fa26b2938efa2059e)
![[Photo] President of the Cuban National Assembly visits President Ho Chi Minh's Mausoleum](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/1/39f1142310fc4dae9e3de4fcc9ac2ed0)






























































































Comment (0)