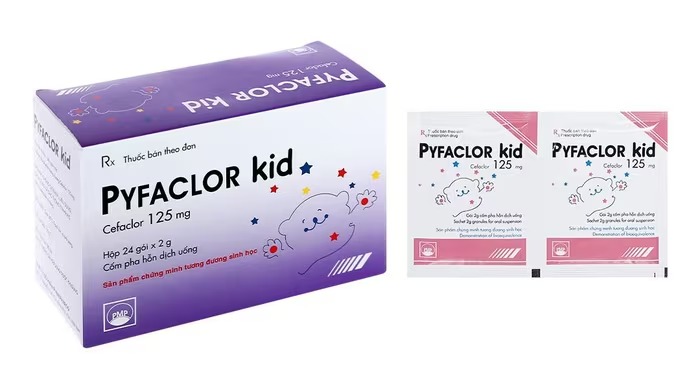
This batch of medicine has circulation registration number VD-26427-17, batch number 410923, production date September 13, 2023, expiry date September 13, 2026, manufactured by Pymepharco Pharmaceutical Joint Stock Company.
The drug is indicated for the treatment of mild to moderate upper and lower respiratory tract infections, including acute otitis media, acute sinusitis, pharyngitis, recurrent tonsillitis, acute bronchitis with secondary infection, pneumonia, exacerbations of chronic bronchitis, uncomplicated urinary tract infections and skin and soft tissue infections.
Illustration photo. |
Two independent testing units took samples of this medicine and the results showed that it did not meet quality standards in terms of properties and quantity, violating level 2 regulations.
In response to this violation, the Drug Administration Department requested Pymepharco Company to stop trading and quarantine all remaining batches of drugs at its facilities, and at the same time report on the drug distribution situation, handle and recall the batches of drugs, pay recall costs and compensate for damages according to the law.
The Ministry of Health 's 2024 report shows that although the rate of substandard drugs tends to decrease, the situation of poor quality drugs, especially in the field of medicinal herbs and oriental medicine, still poses many worrying risks.
Over the past year, the state testing system has tested the quality of more than 43,000 samples of drugs, cosmetics and medicinal materials circulating on the market. Of these, 228 samples did not meet quality standards, accounting for 0.53%.
Although this rate decreased compared to 2019 (1.34%), it was still higher in the medicinal herbs (3.04%) and cosmetics (1.50%) groups than in the modern medicine (0.30%) and oriental medicine (0.40%) groups.
Notably, in 2024, the Ministry of Health discovered 23 samples suspected of being fake drugs and medicinal herbs, including 11 samples of traditional medicine with false labels, not existing on the actual market, and containing active pharmaceutical ingredients such as paracetamol and diclofenac.
Some other active ingredients such as cefixim, cefuroxime, mebendazole, salbutamol, tetracycline are also counterfeited in some localities including Binh Duong, Hue, Soc Trang , Thanh Hoa and Vinh Phuc, places with high demand for drugs, easily becoming targets of counterfeit drugs.
In 2024, the Ministry of Health strictly handled violations, with 65 substandard drug samples being recalled, 10 drug batches being completely recalled, 4 products being voluntarily recalled, and at the same time, 46 administrative penalty decisions were issued with a total amount of over 2.5 billion VND and 18 official dispatches warning about counterfeit drugs and drugs of unknown origin to medical facilities and people.
However, the Ministry of Health assessed that drug quality control has achieved many positive results when the rate of counterfeit drugs has been maintained at a low level, ranging from 0.03% to 0.06% in the past 7 years. This reflects improvements in quality management and awareness of pharmaceutical enterprises.
However, there are still many challenges when the medicinal herb distribution system is still scattered, lacks control standards and is difficult to trace, while the control of cosmetics and oriental medicine sold on the market is not uniform among localities. A part of the population still prefers cheap products of unknown origin, creating conditions for poor quality products to exist and spread in the market.
Faced with this situation, the Ministry of Health recommends that localities continue to strengthen post-inspection, strictly handle violators, and at the same time promote communication to raise public awareness in the use of drugs and medical products of clear origin.
The application of technology such as QR codes and warning software to trace the origin of drugs and medicinal herbs is also considered a potential solution to help effectively and transparently manage the pharmaceutical market in the coming time.
Source: https://baodautu.vn/thu-hoi-khan-mot-san-pham-thuoc-khang-sinh-cho-tre-em-d398224.html





![[Photo] Closing ceremony of the 18th Congress of Hanoi Party Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/17/1760704850107_ndo_br_1-jpg.webp)

























![[Photo] Nhan Dan Newspaper launches “Fatherland in the Heart: The Concert Film”](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/16/1760622132545_thiet-ke-chua-co-ten-36-png.webp)











































































Comment (0)