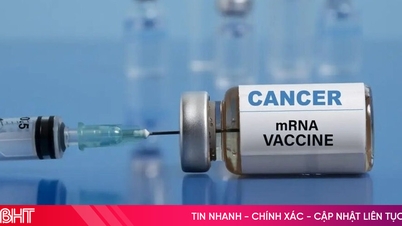
British scientists are to trial the world's first bowel cancer vaccine, which could be available within two years if successful.
The study is being carried out by Cancer Agency UK's Southampton Clinical Trials Unit in collaboration with the Royal Surrey and Queen Elizabeth Hospitals in Australia. Ten trial sites are planned, six in Australia and four in the UK, with 44 patients to be enrolled over an 18-month period.
The vaccine would be given to patients before surgery, helping the body attack the cancerous tumor. This would make the patient's surgery less invasive. Scientists also hope the vaccine could help the immune system respond if the cancer cells recur later.
"This is the first vaccine for a gastrointestinal cancer. We hope that it will be successful, the cancer will completely disappear after treatment," said Dr. Dhillon.
To participate in the trial, patients will undergo endoscopy and tissue samples will be taken to check their health. If eligible, they will be given three doses of the vaccine before surgery to remove the cancer.
After the phase one trial is over, researchers will move on to phase two studies and apply for a license. If the results are positive, researchers believe the vaccine could be licensed for use within two years.
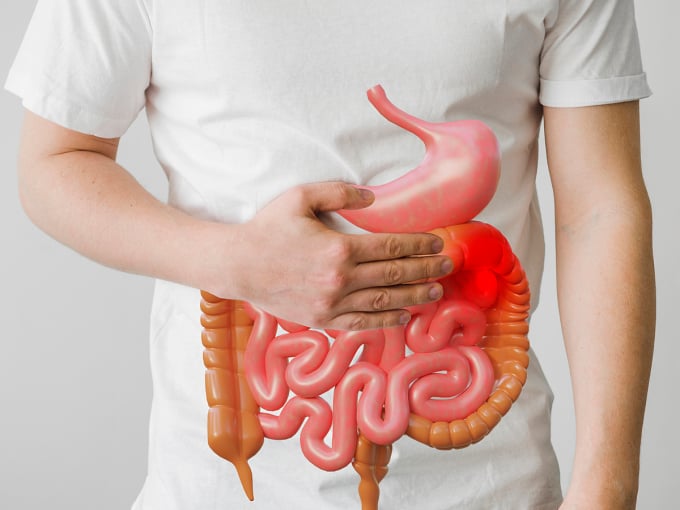
Colon cancer can appear anywhere in the large intestine. Photo: Freepik
Dr. Dhillon calls the vaccine a breakthrough in the fight against cancer. It helps the immune system “chase” the cancer. It will change lives because it allows patients to recover without surgery.
Bowel cancer can occur anywhere in the large intestine, including the colon and rectum. The severity of the disease depends on the size of the tumor, its ability to spread, and the general health of the person.
Clinical symptoms of colon cancer are softer stools, diarrhea, constipation, blood in the stool, anal bleeding, frequent urge to defecate, abdominal pain.
Thuc Linh (According to Clinical Services Journal, Independent, NHS )
Source link


![[Photo] President of the Cuban National Assembly visits President Ho Chi Minh's Mausoleum](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/1/39f1142310fc4dae9e3de4fcc9ac2ed0)




![[Photo] Keep your warehouse safe in all situations](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/1/3eb4eceafe68497989865e7faa4e4d0e)
























![[Photo] Hanoi morning of October 1: Prolonged flooding, people wade to work](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/1/189be28938e3493fa26b2938efa2059e)



























































Comment (0)