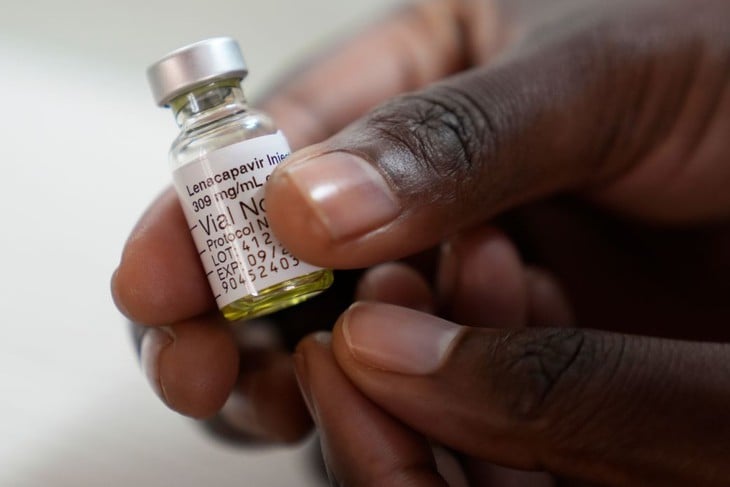
HIV vaccine Lenacapavir.
According to Reuters, this two-dose/yearly injection will be sold in the European Union (EU) under the name Yeytuo, and is allowed to circulate in 27 member countries along with Norway, Iceland and Liechtenstein.
The EC approved the drug as pre-exposure prophylaxis (PrEP), to reduce the risk of sexually transmitted HIV infection in adults and adolescents at high risk of contracting the deadly virus.
Before the drug reaches patients, Gilead needs to finalize agreements on pricing and reimbursement mechanisms with each country's health system.
Lenacapavir proved nearly 100% effective in preventing HIV in large-scale trials last year, raising new hopes of stopping transmission of the virus that infects 1.3 million people a year.
Gilead said its application for EU market approval had been reviewed on an accelerated timetable, and was granted an additional year of market protection.
The company also said it has filed applications for Lenacapavir with regulators in Australia, Brazil, Canada, South Africa and Switzerland and is preparing applications in Argentina, Mexico and Peru.
The World Health Organization last July recommended Lenacapavir as an add-on option for HIV prevention.
Previously, the US Food and Drug Administration (FDA) approved Lenacapavir under the brand name Yeztugo, with a listed price of more than 28,000 USD for a year of treatment, equivalent to two injections.
But some US insurers are delaying coverage of Yeztugo, citing its exorbitant price tag compared to generics. Some analysts predict sales could reach more than $4 billion a year by 2029.
Gilead said it will pursue applications with regulators in low- and middle-income countries.
The company also plans to provide Lenacapavir to up to 2 million people in low-income countries over the next three years, together with the Global Fund to Fight AIDS, Tuberculosis and Malaria, according to Reuters.
tuoitre.vn
Source: https://baolaocai.vn/eu-da-cap-phep-cho-thuoc-tiem-phong-hiv-post880574.html



![[Photo] Hanoi morning of October 1: Prolonged flooding, people wade to work](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/1/189be28938e3493fa26b2938efa2059e)
































![[Photo] President Luong Cuong receives President of the Cuban National Assembly Esteban Lazo Hernandez](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/30/4d38932911c24f6ea1936252bd5427fa)
![[Photo] Panorama of the cable-stayed bridge, the final bottleneck of the Ben Luc-Long Thanh expressway](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/30/391fdf21025541d6b2f092e49a17243f)
![[Photo] The 1st Congress of Phu Tho Provincial Party Committee, term 2025-2030](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/30/1507da06216649bba8a1ce6251816820)




































































Comment (0)