The injectable polio vaccine was rumored to be deadly, a potion from hell before its first large-scale trial, then came under scrutiny due to manufacturing problems.
According to the World Health Organization (WHO), polio is a highly contagious disease that mainly affects young children. The disease attacks the nervous system and can cause paralysis of the spine and respiratory system, and in some cases can be fatal.
In the late 19th and early 20th centuries, polio became the world's most feared disease. A major outbreak in New York City in 1916 killed more than 2,000 people, and a more severe outbreak in the United States in 1952 killed 3,000. Many survivors suffered lifelong disabilities such as leg braces, crutches, wheelchairs, and breathing support devices.
This context created an urgent need for a vaccine, which was only broken through when a group of three scientists successfully cultured polio virus in human tissue in 1949, including John Enders, Thomas Weller and Frederick Robbins, working together at Boston Children's Hospital (USA).
In the early 1950s, American physician Jonas Salk became the first person to successfully develop an injectable polio vaccine (IPV) from inactivated virus. However, before it was approved, the vaccine faced resistance from the community. The reason was that the vaccine would be tested in the field with the participation of more than 1.8 million Americans.
Local politicians worried that the experiment had gone awry, that the shots might cause disease rather than prevent it, and that state officials would be held accountable. Rumors circulated that warehouses across the country were stockpiling small white coffins to hold the bodies of hundreds of thousands of children who had been tested for Salk's "hell potion."
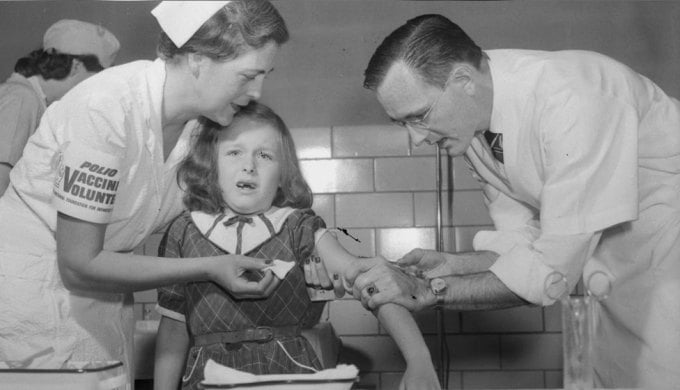
Seven-year-old Mimi Meade grimaces as Dr. Richard Mulvaney injects her with the Salk polio vaccine in 1954. Photo: AP
As the field trial drew near, the rumors grew stronger. Many communities in the states withdrew from the trial, forcing him and the National Foundation for Infantile Paralysis (the nonprofit that supported Salk) to convince each community group to participate.
Salk also relied on the media to convince and reassure the public about the safety of the shot. Time magazine commented: "It is not too much to say that the public trusted the scientist who spoke for himself on the airwaves and in the pages of newspapers. And it is not too much to say that the scientist succeeded."
By April 12, 1955, after a year of testing, the vaccine was declared safe, effective, and well-tolerated. That same day, the vaccine was licensed and began to be used in the community, and even advocated for free distribution to the community, but this was rejected. Salk pledged that the vaccine would be equally accessible, understanding that disease eradication efforts would not be effective without universal, low-cost, or free vaccines.
Six private companies were then licensed to produce and supply vaccines to the public. However, the black market emerged, causing the cost of a dose to increase tenfold, from $2 to $20. This created a conflict when the nonprofit raised money based on community resources, while the price made it accessible only to the wealthy.
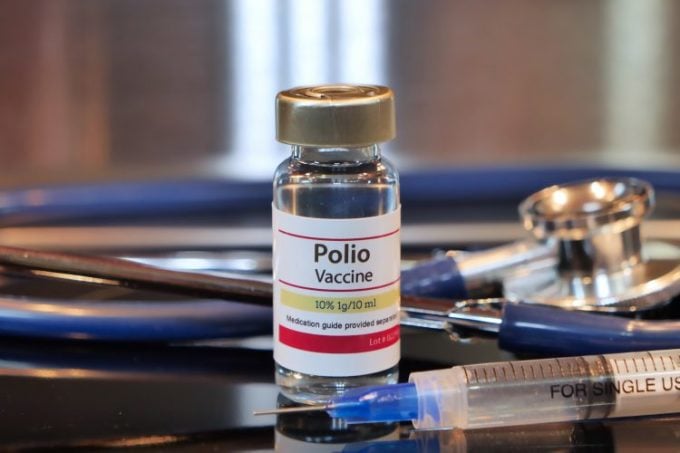
Illustration of the IPV injectable polio vaccine. Photo: Europeanpharmaceuticalreview
Additionally, there were reports of children being hospitalized with polio symptoms after receiving the Salk vaccine. When six vaccinated children died, vaccination was suspended until more information about the vaccine’s safety was known. In this incident, a total of 10 vaccinated children died after contracting polio, and about 200 children developed varying degrees of paralysis.
The U.S. government later determined that the cases originated from Cutter Labs, one of six companies licensed to produce polio vaccine. The company did not follow Salk's detailed process for producing the vaccine, and did not kill the virus during preparation. As a result, children were injected with live virus vaccines. Vaccinations resumed in mid-June under tighter government controls, and the Polio Vaccine Support Act was added.
Within a year, 30 million American children were vaccinated and the number of polio cases was reduced by nearly half. By 1961, the number of polio cases in the US had dropped to 161. In the same year, the second polio vaccine (OPV), developed by virologist Albert Sabin, was approved and later used in Czechoslovakia, Hungary, Cuba, etc. Currently, vaccines continue to be improved for use in polio prevention around the world.
Chile (According to WHO, Time, The Conversation )
Source link





![[Photo] Bustling Mid-Autumn Festival at the Museum of Ethnology](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/4/da8d5927734d4ca58e3eced14bc435a3)
![[Photo] Solemn opening of the 8th Congress of the Central Public Security Party Committee, term 2025-2030](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/4/f3b00fb779f44979809441a4dac5c7df)

























![[Photo] General Secretary To Lam attends the 8th Congress of the Central Public Security Party Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/4/79fadf490f674dc483794f2d955f6045)
























![[VIDEO] Summary of Petrovietnam's 50th Anniversary Ceremony](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/10/4/abe133bdb8114793a16d4fe3e5bd0f12)

![[VIDEO] GENERAL SECRETARY TO LAM AWARDS PETROVIETNAM 8 GOLDEN WORDS: "PIONEER - EXCELLENT - SUSTAINABLE - GLOBAL"](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/7/23/c2fdb48863e846cfa9fb8e6ea9cf44e7)

































Comment (0)