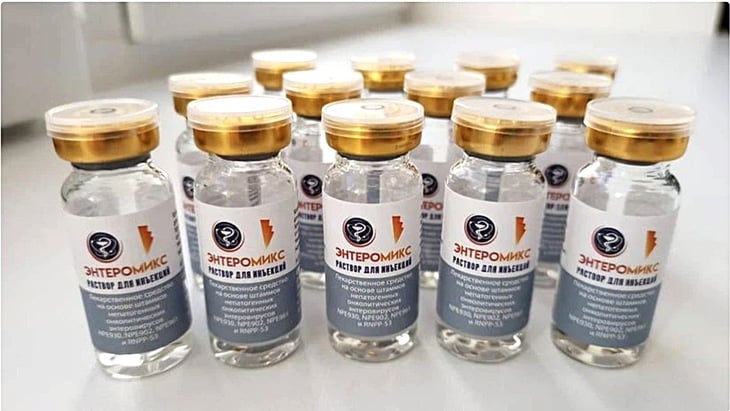
Russia's EnteroMix cancer vaccine is now ready for clinical use - Photo: National Medical Research Center for Radiotherapy, Ministry of Health of the Russian Federation
Russia's Federal Biomedical Agency (FMBA) said EnteroMix is "ready for clinical use" after passing the preclinical stage.
Hope with EnteroMix
The vaccine uses cutting-edge mRNA technology, designed to be personalized for each patient based on tumor RNA. The first version focuses on colorectal cancer, while others are in development for malignant brain tumors and melanoma.
In India Today, molecular oncologist Dr. Yulia Mikhailova said that EnteroMix trains the immune system to recognize and destroy cancer cells using mRNA technology. Unlike traditional vaccines that use weakened viruses, mRNA vaccines deliver genetic instructions for cells to make proteins, thereby triggering an immune response. BIS Research described: "It works by taking a tumor sample, sequencing the RNA, and then creating a customized mRNA vaccine that instructs the body to produce proteins that mimic cancer cells."
The key to EnteroMix is its personalisation: RNA is taken directly from the tumour, sequenced and then made into a tailored vaccine. EnteroMix also incorporates “four harmless viruses” to attack cancer cells and boost immunity, according to Business Standard. Veronika Skvortsova, head of FMBA, said the vaccine has been in pre-clinical testing for years, proving to be safe, highly effective and repeatable.
Reports of EnteroMix trial results have shown impressive but varying results. According to Newsweek magazine, studies have shown "tumor size reductions and growth rates slowing by 60 to 80 percent, depending on the type of disease."
In the phase 1 clinical trial that began in June 2025 with 48 colorectal cancer patients, BIS Research noted: "The results showed that tumors were reduced by 60 - 80% in some cases, and in early-stage patients, the tumors were completely eliminated."
However, Dr. David James Pinato from Imperial College London emphasized: "The fact that a vaccine method is 100% effective in animals does not mean anything." He noted that preclinical results are only in animals, and do not reflect the complexity of the human genome and immunity.
Dr Priya Arora from the Indian Cancer Society added: "This is certainly an exciting result. But until we have long-term data demonstrating that EnteroMix is superior to existing approaches, across a range of patient groups, we must consider this to be a very early-stage study."
In terms of cost, each dose of EnteroMix costs about 300,000 rubles (equivalent to 2,870 USD), but the Russian government has pledged to provide it free of charge to citizens with cancer.
Preventive and therapeutic vaccines
According to Medical Independent, cancer vaccines are currently divided into two groups: preventive vaccines and therapeutic vaccines. Preventive vaccines have proven to be effective. Meanwhile, therapeutic vaccines are still in the experimental stage but have seen breakthroughs.
As of mid-2025, more than 120 clinical trials of RNA vaccines are underway on a variety of cancers, from lung, breast, prostate to brain tumors. Some studies have achieved a 44% reduction in recurrence when combining RNA vaccines with immune checkpoint inhibitors, according to the journal Cancers (Basel). However, cost remains a barrier, at over $100,000 per patient.
mRNA technology, which has exploded with COVID-19, now allows for faster production of personalized vaccines. Process improvements have shortened production times from nine weeks to less than four. In parallel with mRNA, other platforms such as DNA vaccines are also advancing.
To date, the US FDA has approved only three cancer vaccines: Bacillus Calmette-Guérin (BCG) for non-muscle-invasive bladder cancer, Sipuleucel-T (Provenge) for metastatic castration-resistant prostate cancer, and Talimogene laherparepvec (T-VEC, Imlygic) - an oncolytic virus-based vaccine - for the treatment of inoperable advanced melanoma. These vaccines are specific immunotherapy that stimulates the patient's immune system to fight cancer cells and are used under the direction of a specialist.
New advances are changing the landscape. A review published in Nature (2025) notes: “Modern vaccines target tumor-specific antigens (neoantigens) and are showing early evidence of clinical efficacy, particularly in early-stage and pre-cancers when combined with immune checkpoint inhibitor therapy.”
This is the key: instead of using single-agent vaccines for late-stage cancers, current strategies focus on combining vaccines with other therapies in the early stages, when the immune system is still able to mount a strong response.
The race of the "big guys"
Not only Russia, according to Forbes India, "giants" such as BioNTech (Germany), Moderna (USA), CureVac (Germany) are all in phase II or III clinical trials with personalized mRNA vaccines for many types of cancer, from pancreas to lung.
He has launched the Cancer Vaccine Launch Pad program, which is expected to test thousands of patients by 2030. This is a national project of the UK National Health Service (NHS), focusing on personalized cancer vaccines based on mRNA technology.
Source: https://tuoitre.vn/thu-nghiem-vac-xin-ung-thu-nhieu-nuoc-thanh-cong-buoc-dau-20250915101846202.htm



![[Photo] Prime Minister Pham Minh Chinh chairs a meeting of the Government Standing Committee to remove obstacles for projects.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/06/1759768638313_dsc-9023-jpg.webp)


![[Photo] Prime Minister Pham Minh Chinh chaired a meeting of the Steering Committee on the arrangement of public service units under ministries, branches and localities.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/06/1759767137532_dsc-8743-jpg.webp)





























































































Comment (0)