The Drug Administration of Vietnam requests wholesale and retail establishments and pharmacy chain organizations to stop trading, supplying and dispensing the above-mentioned recalled drug batches; medical examination and treatment establishments and drug users to stop prescribing, selling, dispensing and using the above-mentioned recalled drug batches.
According to the Ministry of Health , in 2024, among the samples tested for quality control, substandard drugs accounted for 0.45%, the rate of counterfeit drugs was less than 0.1%, and no counterfeit drugs were detected at medical examination and treatment facilities.
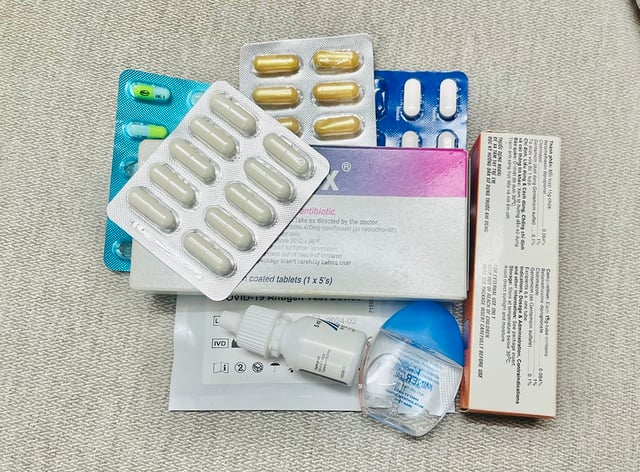
Every year, nearly 38,000 samples of circulating drugs are tested in the country.
PHOTO: NAM SON
Every year, the health sector collects and tests nearly 38,000 samples of drugs circulating on the market; the results show that the rate of substandard drugs has decreased sharply from more than 10% in the early 1990s to less than 2% in recent years.
Regarding ensuring the supply of drugs and medical supplies, the Ministry of Health said that from July 1, 2025, hospitals with autonomy in regular expenditures (autonomy group 2), autonomy in investment expenditures, and regular expenditures (autonomy group 1) will be able to decide on the purchase of drugs and medical equipment from hospital revenues, health insurance expenditures, and other non-state budget sources without having to bid according to the provisions of the Bidding Law.
Current regulations have simplified procedures, increased transparency and improved the efficiency of contractor selection. The above solutions help ensure basic sufficiency of medicines and medical equipment for treatment and health care.
Source: https://thanhnien.vn/khong-phat-hien-thuoc-gia-tai-cac-co-so-kham-chua-benh-185251003180930536.htm





![[Photo] Bustling Mid-Autumn Festival at the Museum of Ethnology](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/4/da8d5927734d4ca58e3eced14bc435a3)
![[Photo] General Secretary To Lam attends the 8th Congress of the Central Public Security Party Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/4/79fadf490f674dc483794f2d955f6045)
![[Photo] Solemn opening of the 8th Congress of the Central Public Security Party Committee, term 2025-2030](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/4/f3b00fb779f44979809441a4dac5c7df)


















































![[VIDEO] Summary of Petrovietnam's 50th Anniversary Ceremony](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/10/4/abe133bdb8114793a16d4fe3e5bd0f12)

![[VIDEO] GENERAL SECRETARY TO LAM AWARDS PETROVIETNAM 8 GOLDEN WORDS: "PIONEER - EXCELLENT - SUSTAINABLE - GLOBAL"](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/7/23/c2fdb48863e846cfa9fb8e6ea9cf44e7)






























Comment (0)