(Dan Tri) - The VNVC vaccine and biological products factory has an investment capital of about 2,000 billion VND, designed and built by Rieckermann Group (Germany) using the world's leading modern technology, aiming at the Net Zero goal. The factory is expected to start construction in 2025, in Long An .
Vietnam Vaccine Joint Stock Company (VNVC) has just signed a contract to design the VNVC Vaccine and Biological Production Factory with Rieckermann Group (Germany) - the world's leading designer in the field of vaccine and pharmaceutical factories.
Rieckermann is a globally reputable corporation with over 130 years of experience in the field of designing pharmaceutical, vaccine and biological production plants worldwide. The unit has designed many vaccine and pharmaceutical production plants of the world's leading corporations such as GSK, MSD, Roche... according to the high standards of GMP pharmaceutical production plants of EU, FDA, PIC/S, WHO.

Mr. Ngo Chi Dung, Chairman of the Board of Directors, General Director of Vietnam Vaccine Joint Stock Company (VNVC) and Mr. Jorge Domingo Guerra, Business Development Director of Rieckermann Group, signed the contract to design the VNVC Vaccine and Biological Production Plant.
To meet the high requirements from VNVC, Rieckermann has optimally used an area of over 26,000m2 to design a modern complex factory. The factory includes areas for vaccine and biological production, animal breeding and research buildings; and other utility areas, all of which meet high, international standards.
In particular, the vaccine and biological product manufacturing plant area must meet the high GMP standards of the EU, FDA, PIC/S and WHO. The specialized building houses experimental animals to conduct pre-clinical and clinical trials for vaccines and biological products according to international standards GLP (Good Laboratory Practice) and AAALAC (International Standards for Assessment and Accreditation of Laboratory Animal Care). These are high-level standards, ensuring the very important legal and humane requirements of this field.

VNVC vaccine and biological product factory is expected to start construction in 2025.
The entire factory is designed according to LEED (Leadership in Energy & Environmental Design) standards. This is a strict international standard for a comprehensive green construction project, protecting the environment, saving energy and moving towards Net Zero emissions following the modern world trend. This will be the first vaccine factory in Vietnam to meet this standard, achieving the goal of reducing Vietnam's total greenhouse gas emissions to zero by 2050.
When put into operation, the VNVC vaccine and biological products factory will mark a new and important milestone in the sustainable development strategy for the Vietnamese vaccine industry, contributing to soon making Vietnam a vaccine-self-sufficient country, ensuring health security, and enhancing the competitiveness of the Vietnamese pharmaceutical industry internationally.
Speaking at the signing ceremony, Mr. Jorge Domingo Guerra, Business Development Director of Rieckermann Group, said that the group will provide VNVC with an innovative, high-class design that fully meets international standards and criteria on safety, efficiency and long-term sustainability, ensuring continuous production of vaccines and important biological products using the world's leading modern technology.
"By leveraging our expertise and experience in technical solutions, we are confident that VNVC's vaccine manufacturing plant will become a pillar of Vietnam in its efforts to ensure the supply of important vaccines and biological products for people in the country and around the world," said Mr. Jorge Domingo Guerra.
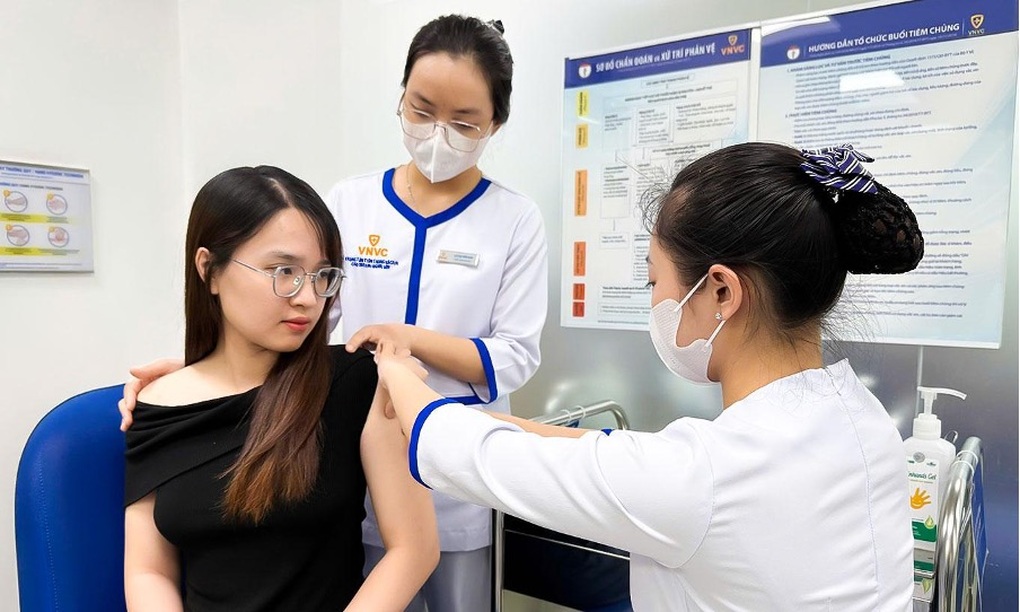
VNVC is the leading prestigious vaccination unit in Vietnam with more than 210 modern centers nationwide, supplying dozens of important vaccines, new vaccines, and new generation vaccines for children and adults.
VNVC Vaccine and Biological Production Plant will be one of the first in Southeast Asia to invest heavily in modern production lines including vial, syringe and pen filling technology; especially the filling and packaging line for vials, syringes and pens using isolator technology. This is the world's most modern sterile production technology, first brought to Vietnam by VNVC to date.
It is expected that when it comes into operation, the VNVC factory will cooperate with the world's leading vaccine manufacturers, participating in each stage of the vaccine and biological product production process, and moving towards receiving technology transfer for independent production.

VNVC and Sanofi signed a cooperation orientation to transfer technology to produce some of Sanofi's vaccines at VNVC factory.
In October 2024, VNVC initially agreed on a cooperation agreement, aiming to transfer technology and produce a number of vaccines from the world's leading pharmaceutical company Sanofi (France) at VNVC's factory, such as flu vaccine and 6-in-1 vaccine, helping Vietnam proactively source high-quality vaccines for children and adults, not just depending on imported sources.
In addition to vaccine production, the VNVC factory complex also prepares modern infrastructure for scientific research and clinical vaccine trials, creating favorable conditions to gather the world's leading experts from within and outside the country to work.
Source: https://dantri.com.vn/xa-hoi/vnvc-moi-tap-doan-duc-thiet-ke-nha-may-vaccine-xanh-quy-mo-2000-ty-dong-20250115161902041.htm







![[Photo] Binh Trieu 1 Bridge has been completed, raised by 1.1m, and will open to traffic at the end of November.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/2/a6549e2a3b5848a1ba76a1ded6141fae)



























































































Comment (0)