SGGP
On June 6, the Ministry of Health issued an official dispatch requesting the People's Committees of provinces and cities across the country to synchronously and drastically deploy solutions to prevent hand, foot and mouth disease, especially focusing on areas with high numbers of cases and at risk of outbreaks.
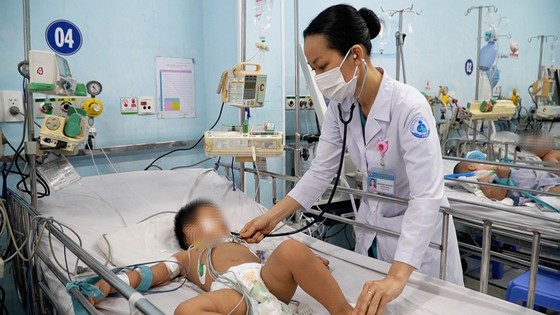 |
| Doctors at Children's Hospital 1 (HCMC) examine a child with hand, foot and mouth disease. Photo: THANH SON |
According to the infectious disease surveillance system, from the beginning of 2023 to now, there have been nearly 9,000 cases of hand, foot and mouth disease nationwide, of which 3 patients died. The number of hand, foot and mouth disease cases has tended to increase rapidly in recent weeks, and the appearance of the Enterovirus (EV71) virus has been recorded, which can cause severe illness in some cases.
On the same day, the Ho Chi Minh City People's Committee issued a document requesting the Department of Health and related departments, district People's Committees and Thu Duc City to synchronously deploy measures to prevent hand, foot and mouth disease according to the instructions of the Ministry of Health.
Regarding the official dispatch of Ho Chi Minh City requesting the Ministry of Health to support two drugs, Immunoglobulin and Phenobarbital, to treat patients with severe hand, foot and mouth disease, on June 6, the Ministry of Health said that there are currently 13 drugs containing Immunoglobulin that have been granted valid circulation registration certificates in Vietnam.
According to the report of the importing establishments on the quantity of Immunoglobulin medicine in stock and the import plan to Vietnam, Duy Anh Pharmaceutical Trading Company Limited imported and currently Cho Ray Hospital has 300 bottles left. It is expected that by the end of July 2023, the manufacturer will supply Vietnam with 5,000-6,000 bottles of this medicine.
For the Human normal Immunoglobulin 100mg/ml medicine imported by Zuellig Pharma Vietnam Company, there are 2,344 boxes of 250ml and 215 boxes of 50ml left. It is expected that in mid-August 2023, the manufacturer will continue to supply Vietnam with 2,000 boxes of 250ml.
Regarding Phenobarbital, there is a type produced by Danapha Pharmaceutical Joint Stock Company, which has a valid circulation registration certificate in Vietnam. Along with that, the Drug Administration of Vietnam (Ministry of Health) has granted permission to Central Pharmaceutical Joint Stock Company CPC1 to import Barbit, a drug that has not yet been registered for circulation in Vietnam, to meet special treatment needs. According to the company's report, there will be 21,000 tubes of medicine (Phenobarbital 200mg/ml) arriving in Vietnam in early July 2023.
To ensure adequate supply of drugs for treatment, the Drug Administration Department requested the Ho Chi Minh City Department of Health to develop and implement a plan for reserving, purchasing, and receiving drugs in accordance with reality, ready to supply enough drugs, ensuring medical examination and treatment. At the same time, urgently direct medical examination and treatment facilities in the area that need to use drugs in a timely manner, proactively contact importing facilities to plan, order, purchase, and reserve drugs in accordance with regulations.
Source



![[Photo] Binh Trieu 1 Bridge has been completed, raised by 1.1m, and will open to traffic at the end of November.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/2/a6549e2a3b5848a1ba76a1ded6141fae)














![[Video] Ministry of Health issues document to rectify medical examination and treatment work](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/10/2/54913f30a9934e18bcbb246c2c85f11d)
















































































Comment (0)