8 food products businesses request to withdraw application
The Food Safety Department ( Ministry of Health ) has just decided to revoke the validity of the certificate of registration of the product declaration of Kingphar super kids health protection food (certificate of registration of product declaration number 13714/2019/DKSP). The product was announced by Vinh Thinh Vuong Production and Trading Company Limited (located in Hung Yen province) and has an official dispatch requesting to withdraw the documents related to the product.
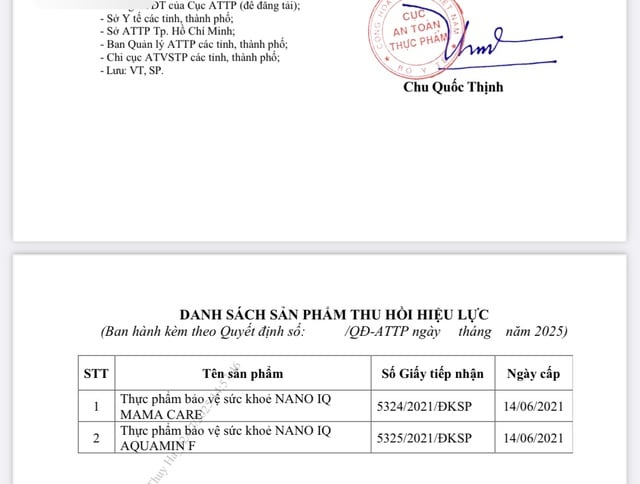
Products with a decision by the Food Safety Department to revoke the product declaration registration certificate
PHOTO: VFA.GOV.VN
The Food Safety Department also revoked the validity of the product declaration registration certificate for the health protection food product Enterovina (product declaration registration certificate number 5803/2019/DKSP). The product was announced by Nguyen Minh Trading and Pharmaceutical Company Limited ( Hanoi ) and requested to withdraw the product-related documents.
4 health protection food products: Nato Thong Huyet Kingphar (6074/2020/DKSP); Menmoren Ginkgo Plus Q10 (6303/2020/DKSP); Digestive enzyme tubes (10160/2020/DKSP); Omega 3-6-9 Plus Q10 Kingphar (3068/2021/DKSP), also had a decision to revoke the validity of the product declaration registration certificate, issued by the Department of Food Safety. 4 products were announced by Kingphar Vietnam Joint Stock Company ( Hung Yen ) and requested to withdraw the product profile.
In addition, the Food Safety Department has decided to revoke the validity of the product registration certificate for 2 health protection foods: Nano IQ mama care (5324/2021/DKSP) and Nano IQ aquamin F (5325/2021/DKSP). 2 products were announced by Nano Pharma Pharmaceutical Joint Stock Company (Hanoi) and requested to withdraw the product profile.
The above products have introductory information: providing vitamins to help children eat well, strengthen immunity; prevent digestive disorders; increase blood circulation, dissolve blood clots; products for pregnant women...
Previously, in June, the Food Safety Department revoked the validity of product declaration registration certificates for many health protection food products.
Source: https://thanhnien.vn/thu-hoi-cong-bo-8-san-pham-bao-ve-suc-khoe-185250703114956898.htm


![[Photo] The 4th meeting of the Inter-Parliamentary Cooperation Committee between the National Assembly of Vietnam and the State Duma of Russia](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/28/9f9e84a38675449aa9c08b391e153183)
![[Photo] High-ranking delegation of the Russian State Duma visits President Ho Chi Minh's Mausoleum](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/28/c6dfd505d79b460a93752e48882e8f7e)


![[Photo] Joy on the new Phong Chau bridge](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/28/b00322b29c8043fbb8b6844fdd6c78ea)

















































































Comment (0)